Reiff Lab research
The neuronal basis of motion vision and visually guided behaviour
Visual motion detection enables the detection of moving objects, predators and prey. Furthermore, the neural analysis of shifting retinal images of the environment underlies the computation of optic flow, navigation and optomotor behaviour. Mathematically, visual motion detection is well described by an algorithmic model, the correlation-type motion detector proposed by Hassenstein and Reichardt in the 1950ies. Today, the Drosophila motion detection circuit represents one of few model systems in which the integrated understanding of all computations performed by all neurons of the completely identified circuitry is coming into reach at the single synapse level. Many labs around the world contribute to this ‘large effort’ in the fly. We employ techniques for the genetic targeting, the functional recording and manipulation of activity to contribute to this effort. Our working horse is optical recording of neural activity in identified neurons employing functional 2PLSM with interlaced virtual reality and genetic Ca2+-indicators.
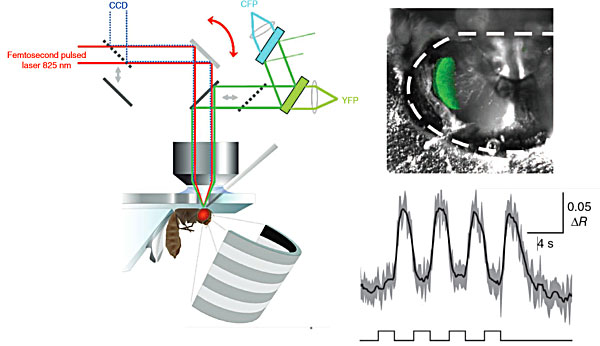
A fly is placed under an objective attached to a two-photon laser-scanning microscope. To reveal cells of the fly visual system, a piece of cuticle is removed from the backside of the fly's head. When the fly visual system is stimulated using a LED arena the activity of cells is be recorded using a calcium indicator (Reiff et al., 2010).
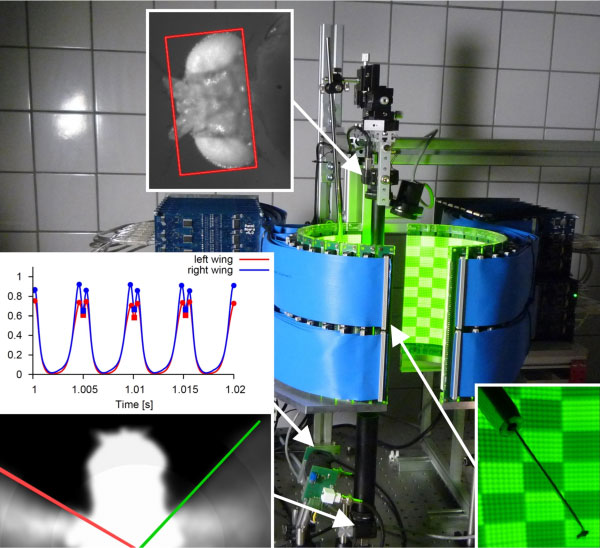
To disclose the neural principles used by the fly to guide visual behaviour we tether flies in the center of a virtual-reality display and present drifting visual patterns to the eyes of the fly in open- or closed loop. Yaw-turning responses (wing beat amplitude and/or head motion) executed by the fly are monitored and analyzed online. Opto- or thermogenetic agents are expressed in specific sets of neurons and controlled by light or heat to activation or inhibition these neurons during sensory processing and the execution of behaviour. Activity in the same and neighbouring neurons has typically been analysed by functional 2PLSM as described above.
A fly is positioned in the centre of a high-speed virtual reality display that is used to present moving patterns to the fly. IR-light, that is invisible to the fly, and CCD-cameras are used to monitor head motion and the envelope of the beating wings. An analog wing beat analyser is used to precisely track the position and motion of the individual beating wings. Differences in the amplitude of the left and right beating wing indicate rotational forces executed by the fly to stabilize or to escape from a specific pattern and motion.
The neuronal basis of colour vision in Drosophila
Colour vision is a major function of the visual system of many animals including insects and humans. The detection of colour allows for example distinguishing ripe fruits from unripe ones and green leaves, a mechanism that gives rise to extensive co-evolution of animals and plants. Furthermore, colour adds another dimension to the image of the environment, allowing for image segmentation where no achromatic contrasts are visible.
Adding chromatic contrast to a photograph of a field cow-wheat (Acker-Wachtelweizen, Liliental bei Freiburg) improves image segmentation and reveals the plant’s visual signal attracting pollinating insects.
To understand the physiology and computational mechanisms of colour vision in fruit flies we combine state of the art genetic techniques for the targeting, functional recording and manipulation of specific populations of neurons in the visual system of the fly. ERG recordings and functional 2PLSM with genetic probes and colour stimulation are used to disclose the contribution of identified neurons to true colour discrimination. Furthermore,
immunolabeling and high-resolution Confocal Microscopy is used to characterize the anatomical organization of selected populations of neurons.
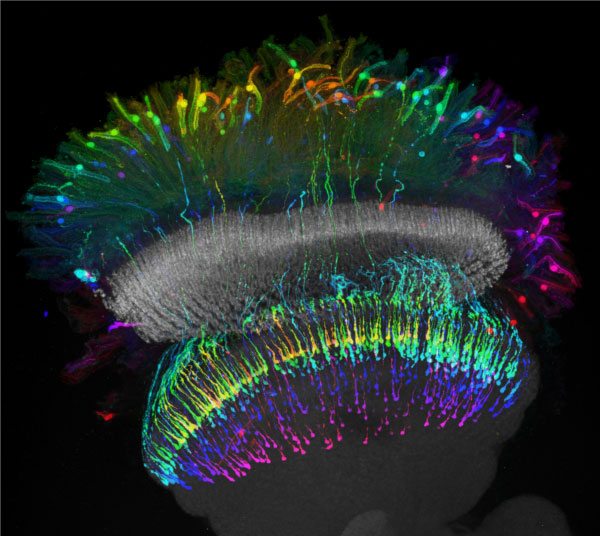
Projection of a confocal stack of photoreceptor neurons expressing targeted Green Fluorescing Protein (GFP). Distal photoreceptors are sending axons to the medulla neuropil where they form presynaptic terminals in a retinotopic manner (colour is employed for coding of depth).
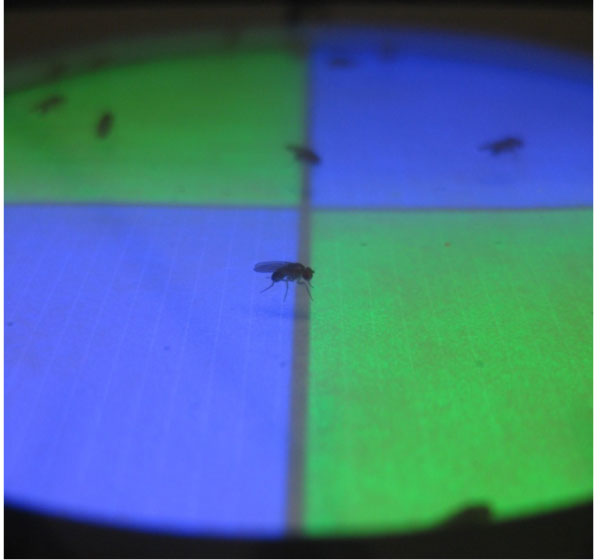
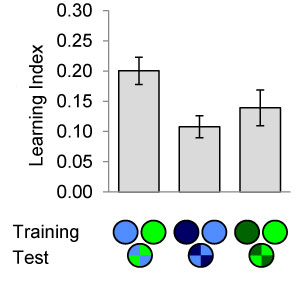
We aim at establishing causal relationships between neural activity in previously characterized neurons and the neural representation of colour in flies. However, flies can not be asked if and how colour is perceived. Thus, we are using a newly developed colour conditioning assay for the quantitative analysis of true colour discrimination based on visual memory. In this computer assisted assay and analysis flies can choose between two colours, one of which has previously been paired with electric shock punishment or sugar reward.
(Left) Flies in a walking arena are trained to associate colour with electric shock or sugar reward. Flies can subsequently be tested for their colour preference in a simultaneous-colour-contrast choice test. (Right) Discriminative colour- but also intensity-memories are analyszed in the behavioural assay (Schnaitmann et al., 2013).